- Clinical
- Physics
- QA
- Dosimetry
- Proton QA & Dosimetry
- Software
- ART-Plan by TheraPanacea
- COMPASS Patient Dose QA
- ImSimQA™
- myQA® Software Physics QA Platform
- myQA® iON for conventional radiotherapy
- myQA® iON for Proton Therapy
- ProSoma – Virtual Simulation
- ProSoma Core – MC plan, check, and logfile analysis
- RadCalc QA Software
- RIT Family of Products
- SagiPlan® HDR & Focal Therapy Planning
- MRI Physics Solutions
- SGRT
- Medical Imaging
- Exterior and Interior Design
- Manufactured by
- Support & Finance
- News
- About
- Contact
HDR Applicators & Accessories

A range of HDR applicators and accessories
GYN & Rectal HDR Applicators
Vaginal/Rectal Cylinder Applicator Set Variable Shielding
 The Vaginal/Rectal Cylinder Applicator Set with Variable Shielding has been designed for use in vaginal and rectal brachytherapy. This set includes 90° and 180° tungsten shielding segments. The Cylinders are available in four different diameters to easily adapt to the individual patients’ anatomy. The SagiPlan® and HDRplus treatment planning systems calculate the effect of the shielding for Co-60 and Ir-192 sources. The result of the shielding is shown in isodose-curves as well as the dose at control-points at organs at risk.
The Vaginal/Rectal Cylinder Applicator Set with Variable Shielding has been designed for use in vaginal and rectal brachytherapy. This set includes 90° and 180° tungsten shielding segments. The Cylinders are available in four different diameters to easily adapt to the individual patients’ anatomy. The SagiPlan® and HDRplus treatment planning systems calculate the effect of the shielding for Co-60 and Ir-192 sources. The result of the shielding is shown in isodose-curves as well as the dose at control-points at organs at risk.
CT/MR Segmented Vaginal Applicator Set

The CT/MR Segmented Vaginal Applicator is designed vaginal and cervical brachytherapy. The four-segment design in multiple widths allows for a range of both lengths as well as diameters to fit a variety of anatomies. The titanium construction with laser welded connections allows for enhanced stability and torque resistance.
CT/MR Miami Applicator Set

The CT/MR Miami Applicator 7-channel design allows for either complete or targeted vaginal and cervical brachytherapy. It enables accurate and reliable treatments in nearly any anatomy with a range of intrauterine angle and stump configurations, various sized build-up caps and optional dome end build-up caps. The single step applicator placement requires minimal intracavitary assembly.
CT/MR Fletcher Applicator Set
 The CT/MR Fletcher Applicator Set generates precise dose distribution to completely encompass the endometrium and the cervix. The CT compatible and MR conditional applicator is made of titanium. This makes it mechanically robust, with minimal artefacts in CT and MR images. Once in position, the thin intrauterine tubes and the ovoid tubes lock together and are secured with tightening screws to remain stable and immobile during treatment.
The CT/MR Fletcher Applicator Set generates precise dose distribution to completely encompass the endometrium and the cervix. The CT compatible and MR conditional applicator is made of titanium. This makes it mechanically robust, with minimal artefacts in CT and MR images. Once in position, the thin intrauterine tubes and the ovoid tubes lock together and are secured with tightening screws to remain stable and immobile during treatment.
CT/MR Fletcher (FSD) Applicator Set
 The CT/MR Fletcher (FSD) Applicator leverages the intrauterine tube and ovoid structure of the Fletcher-Suit-Delclos design but improves usability and flexibility. The connecting bracket is designed with concentric locking knobs that fix the intrauterine tube in place between the ovoids while the lateral positioning remains unrestricted. All materials for this applicator, such as medical grade titanium, have been selected to enable MR conditionality for enhanced planning and structural integrity.
The CT/MR Fletcher (FSD) Applicator leverages the intrauterine tube and ovoid structure of the Fletcher-Suit-Delclos design but improves usability and flexibility. The connecting bracket is designed with concentric locking knobs that fix the intrauterine tube in place between the ovoids while the lateral positioning remains unrestricted. All materials for this applicator, such as medical grade titanium, have been selected to enable MR conditionality for enhanced planning and structural integrity.
CT/MR Henschke Applicator Set
 The CT/MR Henschke Applicator leverages the well-accepted intrauterine tube and ovoid structure of the Henschke design but improves usability and flexibility. The connecting bracket is designed with concentric locking knobs that fix the intrauterine tube in place between the ovoids while the lateral positioning remains unrestricted, providing a high degree of flexibility and adaptation to anatomical conditions. All materials for this applicator, such as medical grade titanium, have been selected to enable MR conditionality for enhanced planning and structural integrity.
The CT/MR Henschke Applicator leverages the well-accepted intrauterine tube and ovoid structure of the Henschke design but improves usability and flexibility. The connecting bracket is designed with concentric locking knobs that fix the intrauterine tube in place between the ovoids while the lateral positioning remains unrestricted, providing a high degree of flexibility and adaptation to anatomical conditions. All materials for this applicator, such as medical grade titanium, have been selected to enable MR conditionality for enhanced planning and structural integrity.
CT/MR Ring Applicator Sets
 The CT/MR Ring Applicator is based on the Stockholm technique for gynaecological brachytherapy. The intrauterine tube and ring can easily be inserted. Once in place, they lock together in a fixed geometry, remaining stable and immobile during treatment. The CT/MR Ring Applicator is available in 30°, 45° and 60° sets, with the angle defined relative to the axis of the applicator. The fixed geometry of the CT/MR Ring Applicator provides a reproducible dose distribution for gynaecological brachytherapy. The combination of a ring and an angled intrauterine tube passing through the centre of the ring results in a targeted dose coverage to the endometrium and cervix.
The CT/MR Ring Applicator is based on the Stockholm technique for gynaecological brachytherapy. The intrauterine tube and ring can easily be inserted. Once in place, they lock together in a fixed geometry, remaining stable and immobile during treatment. The CT/MR Ring Applicator is available in 30°, 45° and 60° sets, with the angle defined relative to the axis of the applicator. The fixed geometry of the CT/MR Ring Applicator provides a reproducible dose distribution for gynaecological brachytherapy. The combination of a ring and an angled intrauterine tube passing through the centre of the ring results in a targeted dose coverage to the endometrium and cervix.
CT/MR Split Ring Applicator Sets
 The CT/MR Split Ring Applicator’s patented design combines the benefits of several other intracavitary applicators, with the ability to be configured as a closed ring or split into four different diameter distances in either symmetric or asymmetric arrangements. The connecting bracket enables simple connection and easy confirmation of diameter settings with three clearly visible identifiers associated with physical detents to lock in the chosen position. The Split Ring Applicator Combination Set includes configurations for 30°, 45° and 60° ‘ring’ angles with a variety of intrauterine tube lengths and disposable or optional reusable build-up caps in different sizes to fit a wide variety of anatomies.
The CT/MR Split Ring Applicator’s patented design combines the benefits of several other intracavitary applicators, with the ability to be configured as a closed ring or split into four different diameter distances in either symmetric or asymmetric arrangements. The connecting bracket enables simple connection and easy confirmation of diameter settings with three clearly visible identifiers associated with physical detents to lock in the chosen position. The Split Ring Applicator Combination Set includes configurations for 30°, 45° and 60° ‘ring’ angles with a variety of intrauterine tube lengths and disposable or optional reusable build-up caps in different sizes to fit a wide variety of anatomies.
CT/MR Ring & Tandem Applicator Sets
 The CT/MR Ring & Tandem Applicator is designed to treat accurately and reliably in nearly any anatomy with a range of ring angles, intrauterine tube lengths, build-up capsizes and an optional rectal retractor to increase the distance to organs at risk and eliminate the need for packing. The single ring tube treats the circumference of the cervix and there is only one connection for efficient placement and procedure. The CT/MR Ring & Tandem Applicator is available in 30°, 45° and 60° configurations to effectively treat a variety of anatomies. The titanium construction with laser welded connections allows for enhanced stability and torque resistance.
The CT/MR Ring & Tandem Applicator is designed to treat accurately and reliably in nearly any anatomy with a range of ring angles, intrauterine tube lengths, build-up capsizes and an optional rectal retractor to increase the distance to organs at risk and eliminate the need for packing. The single ring tube treats the circumference of the cervix and there is only one connection for efficient placement and procedure. The CT/MR Ring & Tandem Applicator is available in 30°, 45° and 60° configurations to effectively treat a variety of anatomies. The titanium construction with laser welded connections allows for enhanced stability and torque resistance.
CT/MR 2/3 Channel Endometrial Applicator Sets
 The CT/MR 2/3 Channel Endometrial Applicator is designed for simplicity, even in the most advanced settings. It enables accurate and complete treatment with multiple lateral intrauterine tube spread distances and the ability to use a single applicator as either a two or three channel set. The single bracket connects all three intrauterine tubes with one lock knob, and the key and lock design ensure proper lateral tube alignment. The CT/MR 2/3 Channel Endometrial Applicator is available in 3 cm or 5 cm configurations, both of which can be used as either two or three channel applicators.
The CT/MR 2/3 Channel Endometrial Applicator is designed for simplicity, even in the most advanced settings. It enables accurate and complete treatment with multiple lateral intrauterine tube spread distances and the ability to use a single applicator as either a two or three channel set. The single bracket connects all three intrauterine tubes with one lock knob, and the key and lock design ensure proper lateral tube alignment. The CT/MR 2/3 Channel Endometrial Applicator is available in 3 cm or 5 cm configurations, both of which can be used as either two or three channel applicators.
CT/MR M.A.C. Interstitial GYN Template Sets
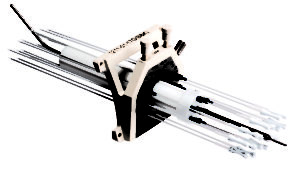 The detachable template and optional intrauterine tube design enable quick setup and simplifies cleaning. Uniquely identified, concentric needle channels enable efficient planning and treatment and the cervical stop enhances repeatability as well as a reduction of uterus perforation. The CT/MR M.A.C. (Mick-Alektiar-Cohen) Interstitial GYN Template features a template design with optional intrauterine tube enabling complete or targeted treatment of the vagina, cervix, endometrium and parametrium. The set is available with 36 needle channels and a variety of diameters.
The detachable template and optional intrauterine tube design enable quick setup and simplifies cleaning. Uniquely identified, concentric needle channels enable efficient planning and treatment and the cervical stop enhances repeatability as well as a reduction of uterus perforation. The CT/MR M.A.C. (Mick-Alektiar-Cohen) Interstitial GYN Template features a template design with optional intrauterine tube enabling complete or targeted treatment of the vagina, cervix, endometrium and parametrium. The set is available with 36 needle channels and a variety of diameters.
Oesophagus & Bronchus HDR Applicators
Oesophagus Applicator Set
 The Oesophagus Applicator Set has been designed for HDR brachytherapy treatment of the oesophagus. The different diameters of the bougie ensure an appropriate radial distance by keeping the oesophagus mucosa away from areas of high dose-gradient. With a 2 mm universal applicator inside the bougie, the source can be placed in a centred radial position relative to the treated tissue. The universal applicator is fixed to the bougie with special adapters. The bougie can be inserted either under fluoroscopy or by using the guidewires
The Oesophagus Applicator Set has been designed for HDR brachytherapy treatment of the oesophagus. The different diameters of the bougie ensure an appropriate radial distance by keeping the oesophagus mucosa away from areas of high dose-gradient. With a 2 mm universal applicator inside the bougie, the source can be placed in a centred radial position relative to the treated tissue. The universal applicator is fixed to the bougie with special adapters. The bougie can be inserted either under fluoroscopy or by using the guidewires
for Seldinger technique under endoscopic examination.
Bronchus Intraluminal Set
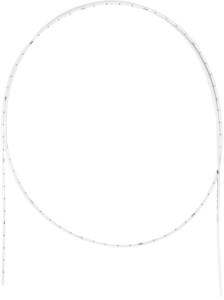 The Bronchus Intraluminal Set has been designed for HDR intraluminal brachytherapy treatments, especially for endobronchial brachytherapy. The small diameter allows easy insertion of the Universal Applicator into a smaller lumen. Since the single-use Universal Applicators are provided sterile, they allow immediate clinical use.
The Bronchus Intraluminal Set has been designed for HDR intraluminal brachytherapy treatments, especially for endobronchial brachytherapy. The small diameter allows easy insertion of the Universal Applicator into a smaller lumen. Since the single-use Universal Applicators are provided sterile, they allow immediate clinical use.
Breast HDR Applicators
Breast Bridge
 The Breast Bridge has been designed for HDR interstitial breast brachytherapy treatments, either for boost treatments or for monotherapy. The template design follows the “Paris System” for homogenous dose Distribution. The separation between the templates is continuously variable up to 180 mm, in order to fit all patient anatomies. The Breast Bridge can be used together with Eckert & Ziegler BEBIG Flexible Catheters Single Leader or plastic needles.
The Breast Bridge has been designed for HDR interstitial breast brachytherapy treatments, either for boost treatments or for monotherapy. The template design follows the “Paris System” for homogenous dose Distribution. The separation between the templates is continuously variable up to 180 mm, in order to fit all patient anatomies. The Breast Bridge can be used together with Eckert & Ziegler BEBIG Flexible Catheters Single Leader or plastic needles.
Breast Bridge with Fixation Buttons
 The Breast Bridge with Fixation Buttons has been designed for HDR interstitial breast brachytherapy treatments, either for boost treatments or for monotherapy. The fixation buttons allow removal of the template, once the Flexible Catheters Single Leader have been implanted. The template’s holes are positioned in equilateral triangles (Paris System) and the separation between the templates is continuously variable between 0 and 180 mm, in order to fit all patient anatomies.
The Breast Bridge with Fixation Buttons has been designed for HDR interstitial breast brachytherapy treatments, either for boost treatments or for monotherapy. The fixation buttons allow removal of the template, once the Flexible Catheters Single Leader have been implanted. The template’s holes are positioned in equilateral triangles (Paris System) and the separation between the templates is continuously variable between 0 and 180 mm, in order to fit all patient anatomies.
Skin HDR Applicators
H.A.M. Applicators (Flabs)
 The Harrison-Anderson-Mick Applicators are used for the treatment of advanced or recurrent colorectal cancer, advanced tumours of pelvis or retroperitoneum, chest wall sarcomas and superficial tumours. They consist of a flexible pad of silicone rubber that is 8 mm thick and available in different sizes. An array of catheters is embedded parallel to each other spaced 10 mm apart while a consistent source-to-tissue distance of 5 mm is maintained.
The Harrison-Anderson-Mick Applicators are used for the treatment of advanced or recurrent colorectal cancer, advanced tumours of pelvis or retroperitoneum, chest wall sarcomas and superficial tumours. They consist of a flexible pad of silicone rubber that is 8 mm thick and available in different sizes. An array of catheters is embedded parallel to each other spaced 10 mm apart while a consistent source-to-tissue distance of 5 mm is maintained.
Valencia Applicator Set
 The Valencia Applicator design allows for non-invasive brachytherapy of skin cancer, which can be performed in a few fractions with high dose rate Ir-192 sources. The tungsten-shielded applicator provides a flat dose profile to the skin to provide a more uniform dose to the target area. The 20 mm and 30 mm versions have different tube lengths and, like all Eckert & Ziegler BEBIG and Mick Radio-Nuclear Instruments HDR Applicators, allow for automatic length verification with the SagiNova® afterloader. If the treatment plan requires a 20 mm applicator, the afterloader measures the length of the connected applicator and checks that it matches the expectation.
The Valencia Applicator design allows for non-invasive brachytherapy of skin cancer, which can be performed in a few fractions with high dose rate Ir-192 sources. The tungsten-shielded applicator provides a flat dose profile to the skin to provide a more uniform dose to the target area. The 20 mm and 30 mm versions have different tube lengths and, like all Eckert & Ziegler BEBIG and Mick Radio-Nuclear Instruments HDR Applicators, allow for automatic length verification with the SagiNova® afterloader. If the treatment plan requires a 20 mm applicator, the afterloader measures the length of the connected applicator and checks that it matches the expectation.
Head & Neck HDR Applicators
Tongue and Mouth Applicator Set
 The Tongue and Mouth Applicator Set is used for the brachytherapy of the tongue and floor of the mouth. The 16G plastic needles are designed to be easily inserted with an obturator ensuring an accurate implant of the target volume. The fixation caps are tolerable for the patient during treatment by covering the needle tips.
The Tongue and Mouth Applicator Set is used for the brachytherapy of the tongue and floor of the mouth. The 16G plastic needles are designed to be easily inserted with an obturator ensuring an accurate implant of the target volume. The fixation caps are tolerable for the patient during treatment by covering the needle tips.
Nasopharynx Applicator Set
 The Nasopharynx Applicator (Rotterdam Type) is designed for intracavitary HDR brachytherapy treatment of nasopharyngeal tumours. It is made of flexible silicone that adapts to the individual nasopharyngeal cavity. The Nasopharynx Applicator is delivered sterile and for single use only. While the flexible catheters are removed, the applicator remains in place for multiple fractions and keeps its fixed position, thus allowing precise and reproducible dose distribution for every single fraction.
The Nasopharynx Applicator (Rotterdam Type) is designed for intracavitary HDR brachytherapy treatment of nasopharyngeal tumours. It is made of flexible silicone that adapts to the individual nasopharyngeal cavity. The Nasopharynx Applicator is delivered sterile and for single use only. While the flexible catheters are removed, the applicator remains in place for multiple fractions and keeps its fixed position, thus allowing precise and reproducible dose distribution for every single fraction.
Prostate HDR Applicators
CT/MR Contour Prostate Template Sets
 The CT/MR Contour Prostate Template facilitates interstitial needles for the treatment of the prostate. Needles of different gauges are securely held in place by colour-coded needle collets associated with the appropriate needle size. They can be positioned and locked individually which allows the reconstruction on the treatment planning system directly after insertion. The numbering/lettering layout of the CT/MR Contour Prostate Template corresponds to the type of stepper being used.
The CT/MR Contour Prostate Template facilitates interstitial needles for the treatment of the prostate. Needles of different gauges are securely held in place by colour-coded needle collets associated with the appropriate needle size. They can be positioned and locked individually which allows the reconstruction on the treatment planning system directly after insertion. The numbering/lettering layout of the CT/MR Contour Prostate Template corresponds to the type of stepper being used.

Applicators & Accessories

Arrange a demo

SagiNova HDR Afterloader
Found what you're looking for or need to discuss your requirements?
Call us today on +44 (0)1743 462694 or email us here
Access your product documents and software downloads via OSL Portal

